Pharmaceutical Cold Rooms & Chambers

±0.1 °C stability. 100 % redundancy. Nationwide delivery in 48 hours.
When drug integrity, patient safety, and regulatory compliance are on the line, temperature fluctuations and the compromises that come with refrigerated shipping containers can have serious ramifications. Polar Leasing’s rental cold chambers and cold rooms give pharmaceutical, biotech, and compounding facilities the tight‑tolerance, GMP‑compliant environment they need, fast.
Proven Compliance & Trust
Independent testing confirms our rooms hold set‑point to within ±0.1 °C, as seen in these validation reports. Every Polar Leasing unit is engineered to satisfy FDA cGMP guidance, GDP best practices, and ISO 9001 quality standards, so your on‑site pharmaceutical chamber meets regulatory expectations.



Pharma Cold Room Solutions
Whether you’re storing monoclonal antibodies, other biologics, or temperature‑sensitive reagents, Polar Leasing offers a turnkey rental that aligns with your SOPs and audit trail.
| Application | How Polar Leasing Helps |
|---|---|
| Compounding Pharmacies | USP <797>/<800> temperature integrity for sterile and hazardous preps—no building modifications required. |
| Blood & Plasma Banks | Stable +4°C and -20°C zones; integrated data logging for FDA inspections. |
| Clinical-Trial Depots | Temporary storage for site initiation or overflow; validated reports satisfy sponsor audits. |
| Research & University Labs | Precise temp and humidity for reagents, media, and specimens. |
| Pharma Distribution Hubs | Buffer inventory during facility upgrades or seasonal peaks; forklift-rated floors handle pallet loads. |

Key Features & Benefits
No two cold room or pharmaceutical chamber needs are identical, but they all demand unwavering reliability. Our standard cold room rentals deliver premium performance out of the gate.
- Fiberglass construction — seamless, patented fiberglass construction offers greater R-value and more consistent temperatures compared to retrofitted shipping containers.
- KE2 precision controls — PID logic maintains target temp to within ±0.1 °C; cloud monitoring & SMS/email alerts included.
- Engineered redundancy — optional redundant refrigeration units protect inventory during maintenance or outage.
- Rapid deployment — fleet staged nationwide; most sites supplied in 24–48 hrs.
- Rental flexibility — weekly, monthly, or long‑term rentals available; scale units as volume or temp requirements change.
Validation Assurance
Polar Leasing’s rental cold chambers and cold rooms offer industry-leading temperature stability—meeting regulatory scrutiny and giving you proof of performance.
IQ / OQ / PQ Documentation
Each unit ships with completed protocols plus a current temperature‑mapping report.
Regulatory Alignment
Designed to support GMP, GDP, FDA 21 CFR §211, and WHO TRS 961 Annex 9 guidance.
Sample Reports (Validation & Mapping)
Available for immediate download: * A820 Validation Report * DT8x10 Validation Report * DT8x20 Validation Report * DT8x20P Validation Report * DT8x20RP Validation Report * L820 Validation Report

Explore Our Pharma‑Ready Rentals
Polar Leasing offers two validated solutions tailored for life‑science applications—ready to ship nationwide in 24‑48 hours:
Pharmaceutical Cold Rooms
Cold rooms that maintain -18 °C to ‑30 °C with KE2 precision control.

Pharmaceutical Cold Chambers
Purposebuilt stability chamber offering precise temperature and humidity profiles for longterm drug studies.

Redundant Walk‑In Freezers
Twin‑refrigeration system delivers 100% backup, safeguarding high‑value inventory during maintenance or power loss.
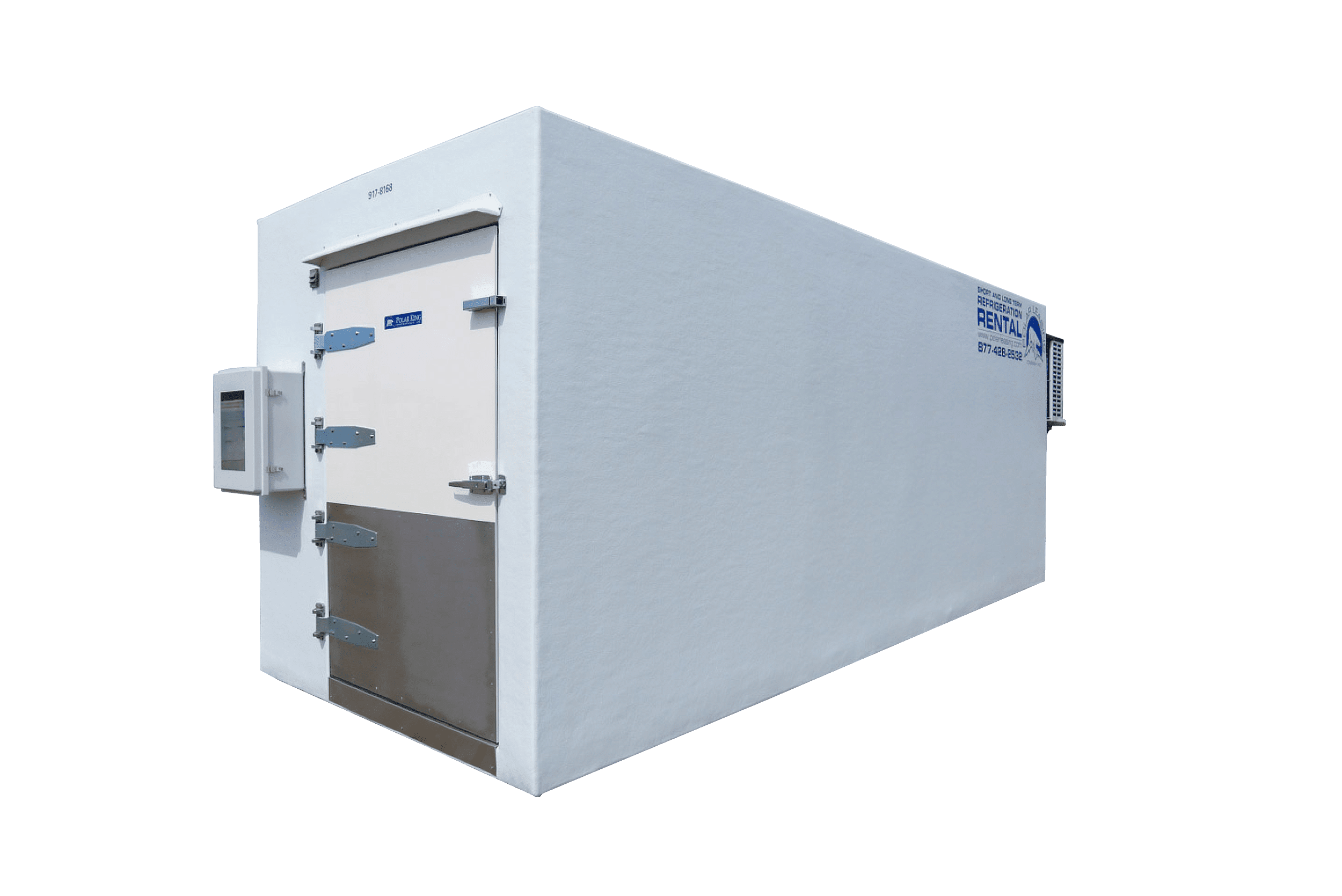
Polar Leasing vs. Container Conversions
Temporary cold storage shouldn’t mean compromised standards. See why purpose‑built stability chambers and cold rooms outclass modular retrofitted shipping containers.
| Polar Leasing Cold Room | Typical Refrigerated Container | |
|---|---|---|
| Construction | Seamless fiberglass, 4" foam core | Steel shell, 2" foam, welded seams |
| Temp Stability | ±0.1°C with KE2 controls | ±2–3°C swing during defrost |
| Redundancy | Standard dual circuits | Single circuit (optional add-on) |
| Floor Load | 5,000 lb/sq ft forklift-rated | 1,500 lb/sq ft |
| Audit Docs | IQ/OQ/PQ + mapping report | Basic performance spec only |
Ready to Protect Your Pharma Products?
Frequently asked questions
1. How fast can you deliver?
We maintain regional fleets; most US locations receive units within 24‑48 hrs of order.
2. What happens if power is lost on‑site?
Dual refrigeration circuits restart automatically. Further, our units’ offer the ability for generator hook‑ups and remote alerts keep product safe.
3. Do you offer month‑to‑month rentals?
Absolutely. Choose weekly, monthly, or multi‑year terms; scale up or down as projects evolve.
